Your Secret Weapon for Boosting Precipitation/Mineralization and/or Hydrate Formation - Part #1
About Scaling - no, not that Scaling!
Amir Mahmoudkhani is one of the foremost experts in the world on the subject of de-scaling, hydrate removal, flow assurance and cementing. For him, the basic toolkit for preventing or accelerating carbonate precipitation & mineralization (i.e., accelerated scaling) and hydrate formation are similar, like two sides of the same coin.
Methods that achieve descaling can work equally well for encouraging scaling by simply altering the conditions. After all, in chemistry, depending upon the application—whether in water treatment, metal recovery, or hydrate formation in cement—sometimes the goal isn’t to dissolve things… it is to make them fall out.
In our case, what we are interested in is dissolved inorganic carbon in water. The water could be the carrier agent post-Carbon Capture, or the hydrating agent in lower-Carbon or CO2 hardened cement.
Total Dissolved Inorganic Carbon =
CO2 + HCO3- + CO32- + H2CO3
carbon dioxide + bicarbonate + carbonate + carbonic acid
To turn all the different forms of dissolved Carbon into Carbonate, there are six factors that are generally used individually or in combination to accelerate Carbonate precipitation and mineralization in most Carbon storage systems:
1. Increase pH
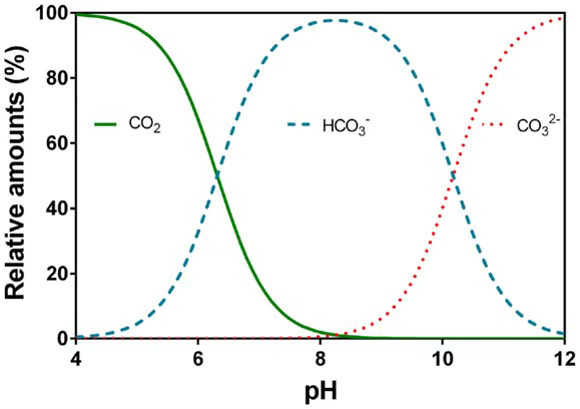
The solubility of many compounds depends on the concentration of hydrogen ions (H⁺) in solution. Changing the pH changes how compounds exist in water—sometimes flipping them from a soluble form to an insoluble one. Higher pH shifts bicarbonates toward carbonate ions (CO₃²⁻), which more readily binds with the metal ions. It also helps additives such as surfactants work better - more on this in a future blog post. Think of pH as a key lever for shifting compounds from “happy to stay dissolved” to “ready to leave the party” (precipitate).
Image credit: Pedersen et al. (2013)
As is evident, increasing CO2 concentration in water increases acidity (lowers pH) through the formation of Carbonic acid.
CO2 + H2O ⇌ H2CO3
Adding alkaline agents help force carbonate and bicarbonate formation.
H2CO3 ⇌ HCO3- + H+
HCO3- ⇌ CO32- + H+
2. Leverage Ideal Gas Law (PV = nRT)
pressure × volume = amount (moles) × ideal gas constant × temperature
As can be seen from the equation, increased heat and/or reduced pressure drives off dissolved CO₂, which reduces bicarbonate solubility and encourages carbonate formation. Heating CO2 infused water from room temperature to 60–80 °C can cause rapid precipitation.
3. Increase CO2 Concentration
CO2 concentration in the carrier medium can be increased by lowering the temperature and/or increasing the pressure, which increases its carrying capacity. When it then comes time for precipitation, the supersaturated liquid can be coaxed into precipitating more CO2 as carbonate.
4. Reduce Water Agitation
Still or slow-moving water lets localized supersaturation to develop, so crystals form and grow more easily.
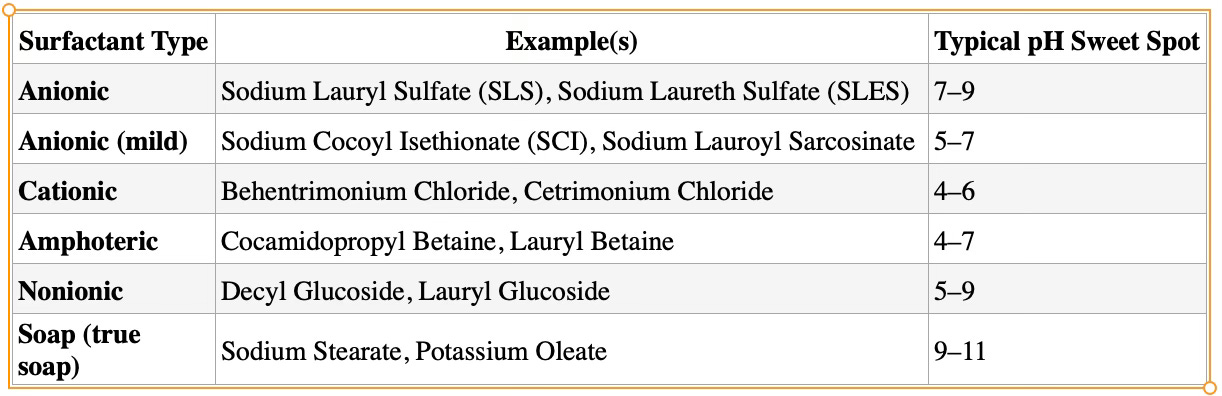
5. Use Surfactants
Change the fluid dynamics of the dissolved CO2 and the carrier so that more of the CO2 can be dissolved in, as well as fall out when required based on how the surfactant is used - especially in combination with pH changes.
Different types of surfactants require different pH levels to operate optimally.
6. Seed the Surface
Provide a “starter crystal” to encourage nucleation. Dropping a piece of chalk, brucite or aragonite into the water can speed up deposition dramatically. This is a specialized variant around adding alkaline agents described towards the end of #1 (Increasing pH).
To the above six well-known levers, we can now add a seventh:
7. Use Mineraccel’s patent-pending special accelerator blend
In combination with one or more of the above levers, Mineraccel’s customized blend does the job faster and better than without.
In a nutshell, this means:
Higher quantum of CO2 precipitated and mineralized
CO2 precipitated and mineralized at a faster rate
Increased strength and other performance characteristics in the case of cement
We will cover this performance differential in detail in part #2 of this post coming soon!